Treatment of Arterial Wall Rupture Induced by a Rotational Atherothrombectomy Device
Abstract
Two types of arterial wall rupture are induced by atherectomy devices: one without bleeding corresponding to pseudoaneurysm formation and one with bleeding. For pseudoaneurysm and rupture with low-flow bleeding, prolonged balloon angioplasty is sufficient. For rupture with high-flow bleeding, a covered stent is indicated. We report 2 cases of vessel preparation where the RotarexTM S Rotational Excisional Atherectomy System (BD) induced arterial wall ruptures and describe how the ruptures were treated in 2 different ways.
J CRIT LIMB ISCHEM 2025:5(3):E36-E40. doi: 10.25270/jcli/CLIG-2400011
Key words: atherectomy, atherothrombectomy, rupture, perforation, peripheral arterial disease, chronic limb-threatening ischemia
Vessel preparation is essential to treat percutaneous peripheral arterial disease (PAD).1 Some lesions, such as chronic total occlusions (CTO), very long and heavily calcified lesions, and in-stent occlusions, are the most challenging.2
Atherectomy devices may be used for vessel preparation to enhance lumen gain, increase vessel compliance, reduce dissection risks, address the need for bailout stenting, and promote drug delivery.3 The RotarexTM S Rotational Excisional Atherectomy System (BD) is a rotational atherothrombectomy device. Complications of atherectomy include distal embolization, dissection, acute occlusion, pseudoaneurysm formation, and arterial wall rupture with bleeding.4 These complications may lead to revascularization failure, amputation, and even death.
We report 2 cases of vessel preparation where the Rotarex S rotational atherothrombectomy device induced arterial wall ruptures and describe how the ruptures were treated in 2 different ways.
Case Report 1
A 44-year-old man presented with chronic limb-threatening ischemia. He had rest pain and first-toe necrosis (Rutherford classification stage 5). Computed tomography angiography (CTA) highlighted thrombosis of a femoropopliteal venous bypass that was performed more than 2 years earlier. An atherothrombectomy device was used to recanalize the bypass (Figure 1). The procedure was complicated by bypass rupture and acute hemorrhage. A 5-mm x 50-mm Viabahn self-expandable covered stent (Gore) was promptly deployed to successfully stop the bleeding.
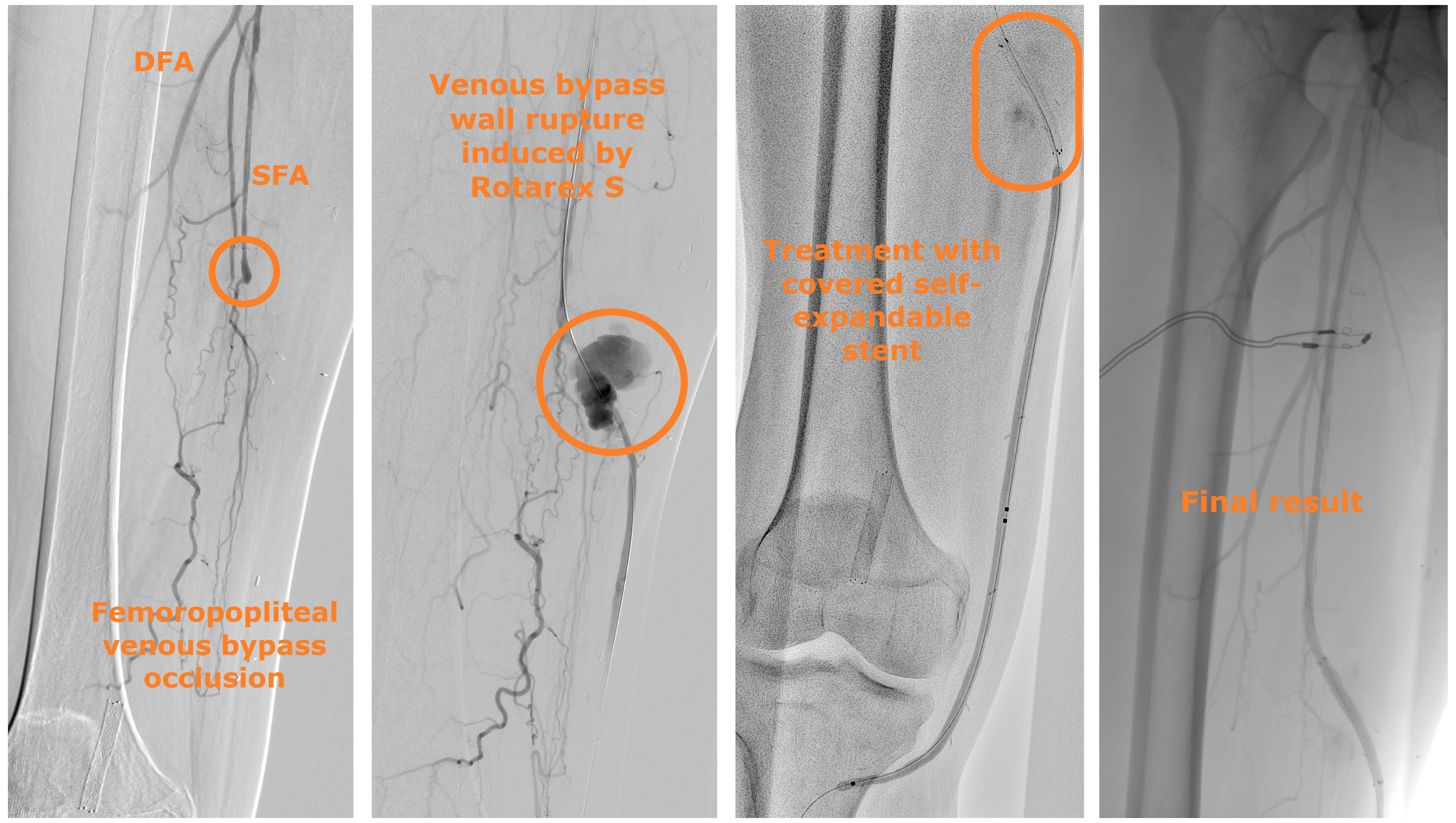
Abbreviations: DFA, deep femoral artery; SFA, superficial femoral artery.
Case Report 2
A 66-year-old woman presented with intermittent claudication in 1 calf (Rutherford classification stage 3). CTA highlighted total occlusion of the whole superficial femoral artery (Global Limb Anatomic Staging System grade 4).5 An atherothrombectomy device was used to prepare the target vessel (Figure 2). The control angiography revealed an arterial wall low-flow leak in the third part of the superficial femoral artery. Prolonged balloon dilation was performed to stop the bleeding. No stent was implanted.

Discussion
Atherectomy is a percutaneous technique in which catheters cut and often suck the atheroma present in the arterial wall.3,6 There are many types of atherectomy devices, including directional, rotational, and orbital atherectomy, and laser ablation.2 In the United States, procedures with atherectomy for PAD have increased by 217% between 2011 and 2019.7
The efficacy of atherectomy on vessel patency, amputation prevention, and mortality is controversial, mainly due to a lack of high-level evidence.6,7 A combination of atherectomy with drug-coated balloon (DCB) angioplasty is promising, especially for complex lesions such as CTO, very long lesions, heavily calcified lesions, and in-stent restenosis.7,8,9
The Rotarex S device is an atherothrombectomy percutaneous catheter intended for arterial occlusions due to either embolic migration or local thrombosis.11 Catheters of 6, 8, and 10F are available, and the device can be used in native arteries and in-stent, venous, or prosthetic bypass. It may be used in acute (<14 days), subacute (<3 months), or chronic (>3 months) situations.11 Adjunct therapies such as balloon angioplasty, DCB, stenting, or thrombolysis are often required.11 There is no randomized controlled trial comparing this system with other atherectomy or percutaneous mechanical thrombectomy devices. There are several single-arm retrospective or observational studies.
The largest cohort studies are the ones reported by Freitas et al in 2017 (525 procedures),12 Artzner et al in 2023 (397 procedures),13 Bulvas et al in 2019 (316 procedures),4 and Wissgot et al in 2008 (265 procedures).14 These studies are summarized in the Table. Amputation or death has not been shown to be related to the use of the system. We found only 1 literature review published in 2020 by Loffroy et al,15 gathering 1844 patients and confirming the safety of the Rotarex S system alone or with adjunctive therapies to treat lower limb occlusive lesions in native arteries, stents, and bypasses. In a recent retrospective study by Huang et al,16 Rotarex S with DCB was compared with Rotarex S alone and with DCB alone for the treatment of long Tosaka class III femoropopliteal in-stent restenosis. The combined therapy showed a primary patency rate at 1 year of 71.7%, Rotarex S alone 27.2%, and DCB alone 35.7%.

Gupta et al reported overall results of rotational atherectomy for PAD with different devices,17 showing a more than 90% technical success rate, 2.2% of distal embolization, 3.8% of perforation, 27.4% of bailout stenting, 0.5% of major amputation at 1 year, and 79.4% of primary patency rate at 1 year. In a paper by Giusca et al, distal embolization was the most common complication, with up to a 4% incidence.18 Arterial wall perforating injuries are less common, with an incidence around 1%.18 Duc et al qualified the risk of perforation induced by Rotarex S as relatively high, with an incidence in native arteries of 9.8%.19 The risk of perforation is 2.3% in in-stent lesions11 and 2.4% in bypass thrombosis.20 Thus it seems that the risk of arterial wall rupture induced by Rotarex S is lower for in-stent occlusive lesions and prosthetic bypass thrombosis.
There are 2 types of arterial wall ruptures: perforation without bleeding or pseudoaneurysm formation and perforation with bleeding. Bleeding may be either high-flow or low-flow. The arterial wall rupture mechanism could be the presence of an intimal flap arising from the arterial wall that is caught by the device in a very fast rotation, resulting in a parietal tear.19 It can be caused or enhanced by the presence of an excentric calcified atherosclerotic plaque pushing the atherectomy catheter against the opposite thin arterial wall.11,21 For cases with excentric plaque, directional atherectomy is probably a better option than rotational atherectomy.
Regarding the Rotarex S itself, the rotating helix inside the device turns at least 40,000 rounds per minute, creating a vortex and strong negative pressure that allows aspiration of thrombi and atherosclerotic debris to be collected in a bag outside the patient’s body. This aspiration is even able to suck in part of the arterial wall, especially in cases of inadequate proximal blood flow,11 thus causing perforation. We therefore recommend avoiding the slightest stop when using the Rotarex S device, advancing carefully with forward and backward passages, and to navigate with the catheter not as slow as with other atherectomy devices. To prevent distal embolization, typically the last centimeter of the occlusion is kept as a natural filter and is crossed only after repeated aspiration of the rest of the lesion.
Another risk factor that may contribute to arterial wall rupture is the position of the guidewire. If it is subintimal, the risk of arterial wall perforation induced by atherectomy devices increases.11,21 Thus atherectomy is contraindicated when the guidewire is subintimal.
The use of atherothrombectomy devices in too-small arteries favors parietal rupture.21 Device failure can cause vessel injury, potentially leading to severe bleeding and death.22 The US Food and Drug Administration has recently reported safety issues with the use of the Rotarex S atherothrombectomy system. The rotating helix portion of the device can completely break when exposed to certain stress, wear, high temperatures, friction, or localized pressure, and BD has adapted the instructions for use.22 A kink-resistant and suitably reinforced sheath, eventually 1 size larger than the Rotarex S catheter, must always be used. The Rotarex S device cannot be used across a vessel bifurcation or curve that results in a curvature of the catheter shaft, resulting in a bend of less than 4 cm in diameter. Enough proximal blood flow has to be maintained to reduce the risk of catheter overheating and blockage. Constant catheter movement has to be performed to limit stress on the device. The Rotarex S system cannot be used in calcified arterial segments. Flushing the catheter several times may be necessary.22
To better evaluate the atherosclerotic plaque characteristics and the guidewire position, intravascular ultrasound (IVUS) may be of help. Sakakura et al reported that IVUS enhances the safety of rotational atherectomy.23 They emphasize the value of IVUS to reduce the occurrence of perforations. Current limitations of IVUS are mainly economic. In our experience, we believe it is useful to perform IVUS before vessel preparation with atherectomy.
When an arterial wall rupture induced by atherectomy occurs, the treatment is driven by the rupture characteristics; our treatment algorithm is shown in Figure 3. When the perforation is not associated with bleeding or is associated with low-flow bleeding, prolonged balloon angioplasty is typically sufficient. When the rupture is associated with high-flow bleeding, prompt deployment of a self-expandable covered stent is indicated.

Conclusion
Pseudoaneurysm formation and arterial wall rupture with bleeding represent distinct types of perforation that may occur with use of rotational atherothrombectomy devices for treating PAD. Treatment of arterial wall rupture induced by atherectomy consists of prolonged balloon angioplasty except when the perforation is associated with high-flow bleeding, where self-expandable covered stent deployment is indicated.
Disclosures
Arnaud Kerzmann, MD; Justine Pudzeis, MD; Evelyne Boesmans, MD; Charlotte Praca, MD; Vlad-Adrian Alexandrescu, MD, PhD; and Vincent Tchana-Sato, MD, PhD, are from the Cardiovascular and Thoracic Surgery Department, Centre Hospitalier Universitaire (CHU), Liège, Belgium. Philippe Brion, MD, is from the Vascular Surgery Department, Clinique Saint-Jean, Brussels, Belgium.
Presented as a poster at the 6th AMP Europe Symposium, Amsterdam, The Netherlands, September 30-October 2, 2024.
Dr Kerzmann is a consultant for BD and Boston Scientific, and has received grant/research support from Medicor, iVascular, Medtronic, Biotronik, DMB Medical, and Abbott.
The authors report no financial relationships or conflicts of interest regarding the content herein.
Manuscript accepted June 3, 2025.
Address for correspondence: Arnaud Kerzmann, MD, Cardiovascular and Thoracic Surgery Department, Centre Hospitalier Universitaire, Avenue de l’Hôpital 1, 4000 Liège, Belgium. Email: akerzmann@chuliege.be

